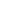ಇತ್ತೀಚೆಗೆ, TÜV SÜD ಚೀನಾ ಗ್ರೂಪ್ (ಇನ್ನು ಮುಂದೆ "TÜV SÜD" ಎಂದು ಉಲ್ಲೇಖಿಸಲಾಗುತ್ತದೆ) FDA 21 CFR ಭಾಗ 11 ರ ಅವಶ್ಯಕತೆಗಳಿಗೆ ಅನುಗುಣವಾಗಿ ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ ದ್ರವ ಸಾರಜನಕ ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆಯ ಎಲೆಕ್ಟ್ರಾನಿಕ್ ದಾಖಲೆಗಳು ಮತ್ತು ಎಲೆಕ್ಟ್ರಾನಿಕ್ ಸಹಿಗಳನ್ನು ಪ್ರಮಾಣೀಕರಿಸಿದೆ. ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ಸ್ವತಂತ್ರವಾಗಿ ಅಭಿವೃದ್ಧಿಪಡಿಸಿದ ಹದಿನಾರು ಉತ್ಪನ್ನ ಪರಿಹಾರಗಳಿಗೆ ಸ್ಮಾರ್ಟ್ಆಂಡ್ ಬಯೋಬ್ಯಾಂಕ್ ಸರಣಿ ಸೇರಿದಂತೆ TÜV SÜD ಅನುಸರಣೆ ವರದಿಯನ್ನು ನೀಡಲಾಯಿತು.
FDA 21 CFR ಭಾಗ 11 ಪ್ರಮಾಣೀಕರಣವನ್ನು ಪಡೆಯುವುದು ಎಂದರೆ ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ LN₂ ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆಯ ಎಲೆಕ್ಟ್ರಾನಿಕ್ ದಾಖಲೆಗಳು ಮತ್ತು ಸಹಿಗಳು ವಿಶ್ವಾಸಾರ್ಹತೆ, ಸಮಗ್ರತೆ, ಗೌಪ್ಯತೆ ಮತ್ತು ಪತ್ತೆಹಚ್ಚುವಿಕೆಯ ಮಾನದಂಡಗಳನ್ನು ಪೂರೈಸುತ್ತವೆ, ಇದರಿಂದಾಗಿ ಡೇಟಾ ಗುಣಮಟ್ಟ ಮತ್ತು ಸುರಕ್ಷತೆಯನ್ನು ಖಚಿತಪಡಿಸುತ್ತದೆ. ಇದು ಯುಎಸ್ ಮತ್ತು ಯುರೋಪ್ನಂತಹ ಮಾರುಕಟ್ಟೆಗಳಲ್ಲಿ ದ್ರವ ಸಾರಜನಕ ಸಂಗ್ರಹಣಾ ವ್ಯವಸ್ಥೆಯ ಪರಿಹಾರಗಳ ಅಳವಡಿಕೆಯನ್ನು ವೇಗಗೊಳಿಸುತ್ತದೆ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ ಅಂತರರಾಷ್ಟ್ರೀಯ ವಿಸ್ತರಣೆಯನ್ನು ಬೆಂಬಲಿಸುತ್ತದೆ.
FDA ಪ್ರಮಾಣೀಕರಣವನ್ನು ಪಡೆದುಕೊಂಡು, HB ಯ ದ್ರವ ಸಾರಜನಕ ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆಯು ಅಂತರಾಷ್ಟ್ರೀಕರಣದ ಹೊಸ ಪ್ರಯಾಣವನ್ನು ಪ್ರಾರಂಭಿಸಿದೆ.
ತೃತೀಯ-ಪಕ್ಷದ ಪರೀಕ್ಷೆ ಮತ್ತು ಪ್ರಮಾಣೀಕರಣದಲ್ಲಿ ಜಾಗತಿಕ ನಾಯಕರಾಗಿರುವ TÜV SÜD, ಕೈಗಾರಿಕೆಗಳಾದ್ಯಂತ ವೃತ್ತಿಪರ ಅನುಸರಣೆ ಬೆಂಬಲವನ್ನು ಒದಗಿಸುವುದರ ಮೇಲೆ ನಿರಂತರವಾಗಿ ಗಮನಹರಿಸುತ್ತದೆ, ಉದ್ಯಮಗಳು ವಿಕಸನಗೊಳ್ಳುತ್ತಿರುವ ನಿಯಮಗಳಿಗೆ ಅನುಗುಣವಾಗಿರಲು ಸಹಾಯ ಮಾಡುತ್ತದೆ. US ಆಹಾರ ಮತ್ತು ಔಷಧ ಆಡಳಿತ (FDA) ಹೊರಡಿಸಿದ ಪ್ರಮಾಣಿತ FDA 21 CFR ಭಾಗ 11, ಎಲೆಕ್ಟ್ರಾನಿಕ್ ದಾಖಲೆಗಳಿಗೆ ಲಿಖಿತ ದಾಖಲೆಗಳು ಮತ್ತು ಸಹಿಗಳಂತೆಯೇ ಕಾನೂನು ಪರಿಣಾಮವನ್ನು ನೀಡುತ್ತದೆ, ಇದು ಎಲೆಕ್ಟ್ರಾನಿಕ್ ಡೇಟಾದ ಸಿಂಧುತ್ವ ಮತ್ತು ವಿಶ್ವಾಸಾರ್ಹತೆಯನ್ನು ಖಚಿತಪಡಿಸುತ್ತದೆ. ಜೈವಿಕ ಔಷಧಗಳು, ವೈದ್ಯಕೀಯ ಸಾಧನಗಳು ಮತ್ತು ಆಹಾರ ಉದ್ಯಮಗಳಲ್ಲಿ ಎಲೆಕ್ಟ್ರಾನಿಕ್ ದಾಖಲೆಗಳು ಮತ್ತು ಸಹಿಗಳನ್ನು ಬಳಸುವ ಸಂಸ್ಥೆಗಳಿಗೆ ಈ ಮಾನದಂಡ ಅನ್ವಯಿಸುತ್ತದೆ.
ಅದರ ಘೋಷಣೆಯ ನಂತರ, ಈ ಮಾನದಂಡವನ್ನು ಪ್ರಪಂಚದಾದ್ಯಂತ ವ್ಯಾಪಕವಾಗಿ ಅಳವಡಿಸಿಕೊಳ್ಳಲಾಗಿದೆ, ಅಮೇರಿಕನ್ ಬಯೋಫಾರ್ಮಾಸ್ಯುಟಿಕಲ್ ಕಂಪನಿಗಳು, ಆಸ್ಪತ್ರೆಗಳು, ಸಂಶೋಧನಾ ಸಂಸ್ಥೆಗಳು ಮತ್ತು ಪ್ರಯೋಗಾಲಯಗಳು ಮಾತ್ರವಲ್ಲದೆ ಯುರೋಪ್ ಮತ್ತು ಏಷ್ಯಾ ಕೂಡ ಇದನ್ನು ಅಳವಡಿಸಿಕೊಂಡಿದೆ. ಎಲೆಕ್ಟ್ರಾನಿಕ್ ದಾಖಲೆಗಳು ಮತ್ತು ಸಹಿಗಳನ್ನು ಅವಲಂಬಿಸಿರುವ ಕಂಪನಿಗಳಿಗೆ, ಸ್ಥಿರವಾದ ಅಂತರರಾಷ್ಟ್ರೀಯ ವಿಸ್ತರಣೆಗೆ FDA 21 CFR ಭಾಗ 11 ಅವಶ್ಯಕತೆಗಳ ಅನುಸರಣೆ ಅತ್ಯಗತ್ಯ, FDA ನಿಯಮಗಳು ಮತ್ತು ಸಂಬಂಧಿತ ಆರೋಗ್ಯ ಮತ್ತು ಸುರಕ್ಷತಾ ಮಾನದಂಡಗಳ ಅನುಸರಣೆಯನ್ನು ಖಚಿತಪಡಿಸುತ್ತದೆ.
ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ ಕ್ರಯೋಬಯೋ ದ್ರವ ಸಾರಜನಕ ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆಯು ಮೂಲಭೂತವಾಗಿ ದ್ರವ ಸಾರಜನಕ ಧಾರಕಗಳಿಗೆ "ಬುದ್ಧಿವಂತ ಮೆದುಳು" ಆಗಿದೆ. ಇದು ಮಾದರಿ ಸಂಪನ್ಮೂಲಗಳನ್ನು ದತ್ತಾಂಶ ಸಂಪನ್ಮೂಲಗಳಾಗಿ ಪರಿವರ್ತಿಸುತ್ತದೆ, ಬಹು ಡೇಟಾವನ್ನು ನೈಜ ಸಮಯದಲ್ಲಿ ಮೇಲ್ವಿಚಾರಣೆ ಮಾಡಲಾಗುತ್ತದೆ, ದಾಖಲಿಸಲಾಗುತ್ತದೆ ಮತ್ತು ಸಂಗ್ರಹಿಸಲಾಗುತ್ತದೆ, ಯಾವುದೇ ವೈಪರೀತ್ಯಗಳಿಗೆ ಎಚ್ಚರಿಕೆ ನೀಡುತ್ತದೆ. ಇದು ತಾಪಮಾನ ಮತ್ತು ದ್ರವ ಮಟ್ಟಗಳ ಸ್ವತಂತ್ರ ದ್ವಿಮಾನ ಮಾಪನವನ್ನು ಹಾಗೂ ಸಿಬ್ಬಂದಿ ಕಾರ್ಯಾಚರಣೆಗಳ ಶ್ರೇಣೀಕೃತ ನಿರ್ವಹಣೆಯನ್ನು ಸಹ ಒಳಗೊಂಡಿದೆ. ಇದರ ಜೊತೆಗೆ, ಇದು ತ್ವರಿತ ಪ್ರವೇಶಕ್ಕಾಗಿ ಮಾದರಿಗಳ ದೃಶ್ಯ ನಿರ್ವಹಣೆಯನ್ನು ಸಹ ಒದಗಿಸುತ್ತದೆ. ಬಳಕೆದಾರರು ಒಂದೇ ಕ್ಲಿಕ್ನಲ್ಲಿ ಹಸ್ತಚಾಲಿತ, ಅನಿಲ-ಹಂತ ಮತ್ತು ದ್ರವ-ಹಂತದ ವಿಧಾನಗಳ ನಡುವೆ ಬದಲಾಯಿಸಬಹುದು, ದಕ್ಷತೆಯನ್ನು ಸುಧಾರಿಸುತ್ತದೆ. ಇದಲ್ಲದೆ, ವ್ಯವಸ್ಥೆಯು IoT ಮತ್ತು BIMS ಮಾದರಿ ಮಾಹಿತಿ ವೇದಿಕೆಯೊಂದಿಗೆ ಸಂಯೋಜಿಸುತ್ತದೆ, ಸಿಬ್ಬಂದಿ, ಉಪಕರಣಗಳು ಮತ್ತು ಮಾದರಿಗಳ ನಡುವೆ ತಡೆರಹಿತ ಸಂಪರ್ಕವನ್ನು ಸಕ್ರಿಯಗೊಳಿಸುತ್ತದೆ. ಇದು ವೈಜ್ಞಾನಿಕ, ಪ್ರಮಾಣೀಕೃತ, ಸುರಕ್ಷಿತ ಮತ್ತು ಪರಿಣಾಮಕಾರಿ ಅಲ್ಟ್ರಾ-ಕಡಿಮೆ ತಾಪಮಾನ ಸಂಗ್ರಹಣೆ ಅನುಭವವನ್ನು ಒದಗಿಸುತ್ತದೆ.
ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ಎಲ್ಲಾ ದೃಶ್ಯಗಳು ಮತ್ತು ಪರಿಮಾಣ ವಿಭಾಗಗಳಿಗೆ ಸೂಕ್ತವಾದ ಸಮಗ್ರವಾದ ಒಂದು-ನಿಲುಗಡೆ ದ್ರವ ಸಾರಜನಕ ಸಂಗ್ರಹ ಪರಿಹಾರವನ್ನು ಅಭಿವೃದ್ಧಿಪಡಿಸಿದೆ, ಮಾದರಿ ಕ್ರಯೋಜೆನಿಕ್ ಸಂಗ್ರಹ ನಿರ್ವಹಣೆಯ ವೈವಿಧ್ಯಮಯ ಅವಶ್ಯಕತೆಗಳ ಮೇಲೆ ಕೇಂದ್ರೀಕರಿಸುತ್ತದೆ. ಪರಿಹಾರವು ವೈದ್ಯಕೀಯ, ಪ್ರಯೋಗಾಲಯ, ಕಡಿಮೆ-ತಾಪಮಾನದ ಸಂಗ್ರಹಣೆ, ಜೈವಿಕ ಸರಣಿಗಳು ಮತ್ತು ಜೈವಿಕ ಸಾರಿಗೆ ಸರಣಿಗಳು ಸೇರಿದಂತೆ ವಿವಿಧ ಸನ್ನಿವೇಶಗಳನ್ನು ಒಳಗೊಂಡಿದೆ ಮತ್ತು ಎಂಜಿನಿಯರಿಂಗ್ ವಿನ್ಯಾಸ, ಮಾದರಿ ಸಂಗ್ರಹಣೆ, ಮಾದರಿ ಮರುಪಡೆಯುವಿಕೆ, ಮಾದರಿ ಸಾಗಣೆ ಮತ್ತು ಮಾದರಿ ನಿರ್ವಹಣೆ ಸೇರಿದಂತೆ ಪೂರ್ಣ-ಪ್ರಕ್ರಿಯೆಯ ಅನುಭವವನ್ನು ಬಳಕೆದಾರರಿಗೆ ಒದಗಿಸುತ್ತದೆ.

FDA 21 CFR ಭಾಗ 11 ಮಾನದಂಡಗಳನ್ನು ಅನುಸರಿಸುವ ಮೂಲಕ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ ಕ್ರಯೋಬಯೋ ದ್ರವ ಸಾರಜನಕ ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆಯು ನಮ್ಮ ಎಲೆಕ್ಟ್ರಾನಿಕ್ ಸಹಿಗಳ ಸಿಂಧುತ್ವ ಮತ್ತು ನಮ್ಮ ಎಲೆಕ್ಟ್ರಾನಿಕ್ ದಾಖಲೆಗಳ ಸಮಗ್ರತೆಗಾಗಿ ಪ್ರಮಾಣೀಕರಿಸಲ್ಪಟ್ಟಿದೆ. ಈ ಅನುಸರಣೆ ಪ್ರಮಾಣೀಕರಣವು ದ್ರವ ಸಾರಜನಕ ಶೇಖರಣಾ ಪರಿಹಾರಗಳ ಕ್ಷೇತ್ರದಲ್ಲಿ ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ ಪ್ರಮುಖ ಸ್ಪರ್ಧಾತ್ಮಕತೆಯನ್ನು ಮತ್ತಷ್ಟು ಹೆಚ್ಚಿಸಿದೆ, ಜಾಗತಿಕ ಮಾರುಕಟ್ಟೆಗಳಲ್ಲಿ ಬ್ರ್ಯಾಂಡ್ನ ವಿಸ್ತರಣೆಯನ್ನು ವೇಗಗೊಳಿಸಿದೆ.
ಬಳಕೆದಾರರನ್ನು ಆಕರ್ಷಿಸಲು ಅಂತರರಾಷ್ಟ್ರೀಯ ರೂಪಾಂತರವನ್ನು ವೇಗಗೊಳಿಸಿ ಮತ್ತು ಜಾಗತಿಕ ಮಾರುಕಟ್ಟೆಗಳ ಸ್ಪರ್ಧಾತ್ಮಕತೆಯನ್ನು ಸುಧಾರಿಸಿ.
ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ಯಾವಾಗಲೂ ಅಂತರರಾಷ್ಟ್ರೀಯ ಕಾರ್ಯತಂತ್ರಕ್ಕೆ ಬದ್ಧವಾಗಿದೆ, ನಿರಂತರವಾಗಿ "ನೆಟ್ವರ್ಕ್ + ಸ್ಥಳೀಕರಣ" ದ್ವಿ ವ್ಯವಸ್ಥೆಯನ್ನು ಉತ್ತೇಜಿಸುತ್ತದೆ. ಅದೇ ಸಮಯದಲ್ಲಿ, ನಾವು ಬಳಕೆದಾರರನ್ನು ಎದುರಿಸಲು ಮಾರುಕಟ್ಟೆ ವ್ಯವಸ್ಥೆಗಳ ಅಭಿವೃದ್ಧಿಯನ್ನು ಬಲಪಡಿಸುವುದನ್ನು ಮುಂದುವರಿಸುತ್ತೇವೆ, ಸಂವಹನ, ಗ್ರಾಹಕೀಕರಣ ಮತ್ತು ವಿತರಣೆಯಲ್ಲಿ ನಮ್ಮ ಸನ್ನಿವೇಶ ಪರಿಹಾರಗಳನ್ನು ಹೆಚ್ಚಿಸುತ್ತೇವೆ.
ಅತ್ಯುತ್ತಮ ಬಳಕೆದಾರ ಅನುಭವವನ್ನು ಸೃಷ್ಟಿಸುವತ್ತ ಗಮನಹರಿಸುತ್ತಾ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ಬಳಕೆದಾರರ ಅಗತ್ಯಗಳಿಗೆ ತ್ವರಿತವಾಗಿ ಪ್ರತಿಕ್ರಿಯಿಸಲು ಸ್ಥಳೀಯ ತಂಡಗಳು ಮತ್ತು ವ್ಯವಸ್ಥೆಗಳನ್ನು ಸ್ಥಾಪಿಸುವ ಮೂಲಕ ಸ್ಥಳೀಕರಣವನ್ನು ಬಲಪಡಿಸುತ್ತದೆ. 2023 ರ ಅಂತ್ಯದ ವೇಳೆಗೆ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ 800 ಕ್ಕೂ ಹೆಚ್ಚು ಪಾಲುದಾರರ ಸಾಗರೋತ್ತರ ವಿತರಣಾ ಜಾಲವನ್ನು ಹೊಂದಿದ್ದು, 500 ಕ್ಕೂ ಹೆಚ್ಚು ಮಾರಾಟದ ನಂತರದ ಸೇವಾ ಪೂರೈಕೆದಾರರೊಂದಿಗೆ ಸಹಕರಿಸಿದೆ. ಏತನ್ಮಧ್ಯೆ, ನಾವು ಯುನೈಟೆಡ್ ಅರಬ್ ಎಮಿರೇಟ್ಸ್, ನೈಜೀರಿಯಾ ಮತ್ತು ಯುನೈಟೆಡ್ ಕಿಂಗ್ಡಮ್ ಅನ್ನು ಕೇಂದ್ರೀಕರಿಸಿದ ಅನುಭವ ಮತ್ತು ತರಬೇತಿ ಕೇಂದ್ರ ವ್ಯವಸ್ಥೆಯನ್ನು ಮತ್ತು ನೆದರ್ಲ್ಯಾಂಡ್ಸ್ ಮತ್ತು ಯುನೈಟೆಡ್ ಸ್ಟೇಟ್ಸ್ನಲ್ಲಿರುವ ಗೋದಾಮು ಮತ್ತು ಲಾಜಿಸ್ಟಿಕ್ಸ್ ಕೇಂದ್ರ ವ್ಯವಸ್ಥೆಯನ್ನು ಸ್ಥಾಪಿಸಿದ್ದೇವೆ. ನಾವು ಯುಕೆಯಲ್ಲಿ ನಮ್ಮ ಸ್ಥಳೀಕರಣವನ್ನು ಆಳಗೊಳಿಸಿದ್ದೇವೆ ಮತ್ತು ಕ್ರಮೇಣ ಜಾಗತಿಕವಾಗಿ ಈ ಮಾದರಿಯನ್ನು ಪುನರಾವರ್ತಿಸುತ್ತಿದ್ದೇವೆ, ನಮ್ಮ ಸಾಗರೋತ್ತರ ಮಾರುಕಟ್ಟೆ ವ್ಯವಸ್ಥೆಯನ್ನು ನಿರಂತರವಾಗಿ ಬಲಪಡಿಸುತ್ತಿದ್ದೇವೆ.
ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ಹೊಸ ಉತ್ಪನ್ನಗಳ ವಿಸ್ತರಣೆಯನ್ನು ವೇಗಗೊಳಿಸುತ್ತಿದೆ, ಪ್ರಯೋಗಾಲಯ ಉಪಕರಣಗಳು, ಉಪಭೋಗ್ಯ ವಸ್ತುಗಳು ಮತ್ತು ಸ್ಮಾರ್ಟ್ ಔಷಧಾಲಯಗಳು ಸೇರಿದಂತೆ ನಮ್ಮ ಸನ್ನಿವೇಶ ಪರಿಹಾರಗಳ ಸ್ಪರ್ಧಾತ್ಮಕತೆಯನ್ನು ಹೆಚ್ಚಿಸುತ್ತಿದೆ. ಜೀವ ವಿಜ್ಞಾನ ಬಳಕೆದಾರರಿಗೆ, ನಮ್ಮ ಸೆಂಟ್ರಿಫ್ಯೂಜ್ಗಳು ಯುರೋಪ್ ಮತ್ತು ಅಮೆರಿಕಾದಲ್ಲಿ ಪ್ರಗತಿ ಸಾಧಿಸಿವೆ, ನಮ್ಮ ಫ್ರೀಜ್ ಡ್ರೈಯರ್ಗಳು ಏಷ್ಯಾದಲ್ಲಿ ಮೊದಲ ಆರ್ಡರ್ಗಳನ್ನು ಪಡೆದಿವೆ ಮತ್ತು ನಮ್ಮ ಜೈವಿಕ ಸುರಕ್ಷತಾ ಕ್ಯಾಬಿನೆಟ್ಗಳು ಪೂರ್ವ ಯುರೋಪ್ ಮಾರುಕಟ್ಟೆಯನ್ನು ಪ್ರವೇಶಿಸಿವೆ. ಏತನ್ಮಧ್ಯೆ, ನಮ್ಮ ಪ್ರಯೋಗಾಲಯದ ಉಪಭೋಗ್ಯ ವಸ್ತುಗಳನ್ನು ಏಷ್ಯಾ, ಉತ್ತರ ಅಮೆರಿಕಾ ಮತ್ತು ಯುರೋಪ್ನಲ್ಲಿ ಸಾಧಿಸಲಾಗಿದೆ ಮತ್ತು ಪುನರಾವರ್ತಿಸಲಾಗಿದೆ. ವೈದ್ಯಕೀಯ ಸಂಸ್ಥೆಗಳಿಗೆ, ಸೌರ ಲಸಿಕೆ ಪರಿಹಾರಗಳ ಜೊತೆಗೆ, ಔಷಧೀಯ ರೆಫ್ರಿಜರೇಟರ್ಗಳು, ರಕ್ತ ಸಂಗ್ರಹ ಘಟಕಗಳು ಮತ್ತು ಉಪಭೋಗ್ಯ ವಸ್ತುಗಳು ಸಹ ವೇಗವಾಗಿ ಅಭಿವೃದ್ಧಿ ಹೊಂದುತ್ತಿವೆ. ಅಂತರರಾಷ್ಟ್ರೀಯ ಸಂಸ್ಥೆಗಳೊಂದಿಗೆ ನಿರಂತರ ಸಂವಹನದ ಮೂಲಕ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ಪ್ರಯೋಗಾಲಯ ನಿರ್ಮಾಣ, ಪರಿಸರ ಪರೀಕ್ಷೆ ಮತ್ತು ಕ್ರಿಮಿನಾಶಕ ಸೇರಿದಂತೆ ಸೇವೆಗಳನ್ನು ಒದಗಿಸುತ್ತದೆ, ಹೊಸ ಬೆಳವಣಿಗೆಯ ಅವಕಾಶಗಳನ್ನು ಸೃಷ್ಟಿಸುತ್ತದೆ.
2023 ರ ಅಂತ್ಯದ ವೇಳೆಗೆ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ನ 400 ಕ್ಕೂ ಹೆಚ್ಚು ಮಾದರಿಗಳನ್ನು ವಿದೇಶಗಳಲ್ಲಿ ಪ್ರಮಾಣೀಕರಿಸಲಾಗಿದೆ ಮತ್ತು ಜಿಂಬಾಬ್ವೆ, ಡೆಮಾಕ್ರಟಿಕ್ ರಿಪಬ್ಲಿಕ್ ಆಫ್ ಕಾಂಗೋ, ಇಥಿಯೋಪಿಯಾ ಮತ್ತು ಲೈಬೀರಿಯಾದಲ್ಲಿನ ಹಲವಾರು ಪ್ರಮುಖ ಯೋಜನೆಗಳಿಗೆ ಹಾಗೂ ಚೀನಾ-ಆಫ್ರಿಕಾ ಯೂನಿಯನ್ ಸೆಂಟರ್ಸ್ ಆಫ್ ಡಿಸೀಸ್ ಕಂಟ್ರೋಲ್ (CDC) ಯೋಜನೆಗೆ ಯಶಸ್ವಿಯಾಗಿ ತಲುಪಿಸಲಾಗಿದೆ, ಇದು ವಿತರಣಾ ಕಾರ್ಯಕ್ಷಮತೆಯ ಸುಧಾರಣೆಯನ್ನು ಪ್ರದರ್ಶಿಸುತ್ತದೆ. ನಮ್ಮ ಉತ್ಪನ್ನಗಳು ಮತ್ತು ಪರಿಹಾರಗಳನ್ನು 150 ಕ್ಕೂ ಹೆಚ್ಚು ದೇಶಗಳು ಮತ್ತು ಪ್ರದೇಶಗಳಲ್ಲಿ ವ್ಯಾಪಕವಾಗಿ ಅಳವಡಿಸಿಕೊಳ್ಳಲಾಗಿದೆ. ಅದೇ ಸಮಯದಲ್ಲಿ, ನಾವು ವಿಶ್ವ ಆರೋಗ್ಯ ಸಂಸ್ಥೆ (WHO) ಮತ್ತು UNICEF ಸೇರಿದಂತೆ 60 ಕ್ಕೂ ಹೆಚ್ಚು ಅಂತರರಾಷ್ಟ್ರೀಯ ಸಂಸ್ಥೆಗಳೊಂದಿಗೆ ದೀರ್ಘಕಾಲೀನ ಸಹಕಾರವನ್ನು ಕಾಯ್ದುಕೊಂಡಿದ್ದೇವೆ.
ಜಾಗತಿಕ ವಿಸ್ತರಣೆಯ ನಮ್ಮ ಪ್ರಯಾಣದಲ್ಲಿ ನಾವೀನ್ಯತೆಯ ಮೇಲೆ ನಾವು ಗಮನಹರಿಸುತ್ತಿರುವುದರಿಂದ FDA 21 CFR ಭಾಗ 11 ಪ್ರಮಾಣೀಕರಣವನ್ನು ಪಡೆಯುವುದು ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ಗೆ ಮಹತ್ವದ ಮೈಲಿಗಲ್ಲು. ಇದು ನಾವೀನ್ಯತೆಯ ಮೂಲಕ ಬಳಕೆದಾರರ ಅಗತ್ಯಗಳನ್ನು ಪೂರೈಸುವ ನಮ್ಮ ಬದ್ಧತೆಯನ್ನು ಸಹ ಪ್ರದರ್ಶಿಸುತ್ತದೆ. ಮುಂದೆ ನೋಡುತ್ತಾ, ಹೈಯರ್ ಬಯೋಮೆಡಿಕಲ್ ನಮ್ಮ ಬಳಕೆದಾರ-ಕೇಂದ್ರಿತ ನಾವೀನ್ಯತೆ ವಿಧಾನವನ್ನು ಮುಂದುವರಿಸುತ್ತದೆ, ಪ್ರದೇಶಗಳು, ಚಾನಲ್ಗಳು ಮತ್ತು ಉತ್ಪನ್ನ ವರ್ಗಗಳಲ್ಲಿ ನಮ್ಮ ಜಾಗತಿಕ ಕಾರ್ಯತಂತ್ರದ ನಿಯೋಜನೆಯನ್ನು ಮುಂದುವರಿಸುತ್ತದೆ. ಸ್ಥಳೀಯ ನಾವೀನ್ಯತೆಗೆ ಒತ್ತು ನೀಡುವ ಮೂಲಕ, ಬುದ್ಧಿಮತ್ತೆಯ ಮೂಲಕ ಅಂತರರಾಷ್ಟ್ರೀಯ ಮಾರುಕಟ್ಟೆಗಳನ್ನು ಅನ್ವೇಷಿಸುವ ಗುರಿಯನ್ನು ನಾವು ಹೊಂದಿದ್ದೇವೆ.
ಪೋಸ್ಟ್ ಸಮಯ: ಜುಲೈ-15-2024